Introduction to Peptides
ALL ARTICLES AND PRODUCT INFORMATION PROVIDED ON THIS WEBSITE ARE FOR INFORMATIONAL AND EDUCATIONAL PURPOSES ONLY. The products offered on this website are furnished for in-vitro studies only. In-vitro studies (Latin: in glass) are performed outside of the body. These products are not medicines or drugs and have not been approved by the FDA to prevent, treat or cure any medical condition, ailment or disease. Bodily introduction of any kind into humans or animals is strictly forbidden by law.

What is a Peptide?
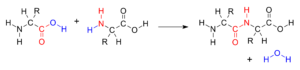
A peptide is a chemical compound that naturally occurs and consists of two or more amino acids linked by peptide bonds. A peptide bond is a covalent bond created when the carboxyl group (C-terminus) of one amino acid combines with the amino group (N-terminus) of another amino acid during a condensation reaction, where a water molecule is released. The bond formed is a CO-NH bond, constituting a peptide, or amide, molecule. Notably, all peptide bonds are also classified as amide bonds. The term "peptide" originates from πέσσειν, a Greek word that translates to "to digest." Peptides play a crucial role in nature and biochemistry, with thousands of different peptides found naturally in the human body and animals. Furthermore, the continual discovery and synthesis of new peptides in laboratories underscore the ongoing advancements and potential in peptide research for health and pharmaceutical development.
How Are Peptides Formed?
Peptides can be created naturally within the body or synthetically in the laboratory. Naturally, the body produces certain types of peptides, including ribosomal and non-ribosomal peptides. In contrast, modern peptide synthesis methods in laboratories can generate an almost unlimited variety of peptides. Techniques such as liquid phase peptide synthesis and solid phase peptide synthesis are employed to craft these peptides. Although liquid phase peptide synthesis offers certain benefits, solid phase peptide synthesis has become the predominant method used in contemporary peptide synthesis practices. Further details on peptide synthesis are available in specialized literature.
The first synthetic peptide was discovered in 1901 by Emil Fischer in collaboration with Ernest Fourneau. Oxytocin, the first polypeptide, was synthesized in 1953 by Vincent du Vigneaud.
Peptide Terminology
Peptides are commonly categorized based on the number of amino acids they contain. A peptide made up of just two amino acids is called a “dipeptide.” Similarly, a peptide composed of three amino acids is known as a “tripeptide.” Peptides that contain a relatively small number of amino acids, generally fewer than ten, are termed “oligopeptides.” In contrast, “polypeptides” typically consist of ten or more amino acids. Peptides that include more than 40-50 amino acids are often classified as proteins.
The distinction between peptides and proteins primarily hinges on the number of amino acids, but there are exceptions. For instance, certain longer peptides are recognized as proteins (such as amyloid beta), and some smaller proteins are sometimes referred to as peptides (like insulin).
Classification of Peptides
Peptides are classified into several types based on their production mechanisms. One key class is ribosomal peptides, which are synthesized from the translation of mRNA. These peptides often serve as hormones and signaling molecules, including tachykinin peptides, vasoactive intestinal peptides, opioid peptides, pancreatic peptides, and calcitonin peptides. Some ribosomal peptides, such as microcins, function as antibiotics and are produced by specific organisms. Ribosomal peptides typically undergo proteolysis, which involves the breakdown of proteins into smaller peptides or amino acids, to achieve their mature form.
On the other hand, nonribosomal peptides are synthesized by specific peptide-synthesizing enzymes, rather than by ribosomes. These peptides are often cyclic, although linear forms are also common, and are known for their complex structures. Nonribosomal peptides are prevalent in plants, fungi, and unicellular organisms, with glutathione being the most notable example, crucial for antioxidant defenses in aerobic organisms.
Milk peptides are another class, derived from milk proteins either through enzymatic breakdown by digestive enzymes or by proteinases from lactobacilli during milk fermentation. Peptones, which are peptides obtained from the proteolytic digestion of animal milk or meat, are used in laboratories as growth nutrients for bacteria and fungi.
Additionally, peptide fragments are typically the result of enzymatic degradation in controlled laboratory settings, but they can also occur naturally due to environmental degradation. These classifications highlight the diverse origins and functions of peptides in biological systems.
Important Peptide Terms
Understanding some fundamental peptide-related terms is essential for grasping concepts related to peptides, their synthesis, and their application in research and experimentation:
- Amino Acids: These are the building blocks of peptides. An amino acid is a molecule that includes both amine and carboxyl functional groups. Alpha-amino acids specifically are used to construct peptides.
- Cyclic Peptides: These peptides have a ring structure rather than a straight chain. Examples include melanotan-2 and PT-141 (Bremelanotide), which are known for their applications in medical research.
- Peptide Sequence: This term refers to the specific order of amino acid residues linked by peptide bonds within a peptide.
- Peptide Bond: A covalent bond formed between two amino acids when the carboxyl group of one amino acid reacts with the amino group of another, releasing a molecule of water during this condensation reaction.
- Peptide Mapping: This analytical technique is used to identify or verify the amino acid sequence of peptides or proteins. It involves breaking down the peptide or protein with enzymes and analyzing the resulting amino acid or nucleotide sequences.
- Peptide Mimetics: These are molecules that mimic the biological activity of hormones, cytokines, enzyme substrates, viruses, or other biomolecules. They can be naturally occurring peptides, synthetically modified peptides, or other molecules designed to fulfill similar functions.
- Peptide Fingerprint: This chromatographic pattern is obtained by partially hydrolyzing a peptide to break it into fragments, followed by two-dimensional mapping of these fragments.
- Peptide Library: A collection of numerous peptides that feature a systematic variation of amino acids. These libraries are crucial for protein studies and have extensive applications in biochemical and pharmaceutical research. Solid phase peptide synthesis is the primary method used to create peptide libraries.
