ALL ARTICLES AND PRODUCT INFORMATION PROVIDED ON THIS WEBSITE ARE FOR INFORMATIONAL AND EDUCATIONAL PURPOSES ONLY. The products offered on this website are furnished for in-vitro studies only. In-vitro studies (Latin: in glass) are performed outside of the body. These products are not medicines or drugs and have not been approved by the FDA to prevent, treat or cure any medical condition, ailment or disease. Bodily introduction of any kind into humans or animals is strictly forbidden by law.

Peptides can be created naturally within the body or synthetically in the laboratory. Naturally, the body produces certain types of peptides, including ribosomal and non-ribosomal peptides. In contrast, modern peptide synthesis methods in laboratories can generate an almost unlimited variety of peptides. Techniques such as liquid phase peptide synthesis and solid phase peptide synthesis are employed to craft these peptides. Although liquid phase peptide synthesis offers certain benefits, solid phase peptide synthesis has become the predominant method used in contemporary peptide synthesis practices. Further details on peptide synthesis are available in specialized literature.
The first synthetic peptide was discovered in 1901 by Emil Fischer in collaboration with Ernest Fourneau. Oxytocin, the first polypeptide, was synthesized in 1953 by Vincent du Vigneaud.
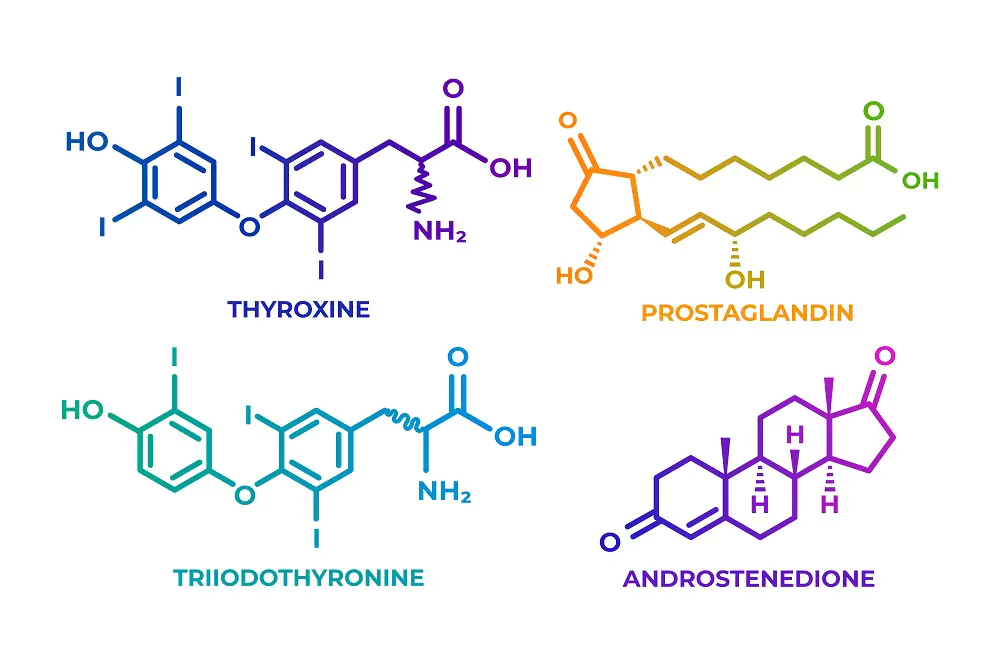
Peptides are classified into several types based on their production mechanisms. One key class is ribosomal peptides, which are synthesized from the translation of mRNA. These peptides often serve as hormones and signaling molecules, including tachykinin peptides, vasoactive intestinal peptides, opioid peptides, pancreatic peptides, and calcitonin peptides. Some ribosomal peptides, such as microcins, function as antibiotics and are produced by specific organisms. Ribosomal peptides typically undergo proteolysis, which involves the breakdown of proteins into smaller peptides or amino acids, to achieve their mature form.
On the other hand, nonribosomal peptides are synthesized by specific peptide-synthesizing enzymes, rather than by ribosomes. These peptides are often cyclic, although linear forms are also common, and are known for their complex structures. Nonribosomal peptides are prevalent in plants, fungi, and unicellular organisms, with glutathione being the most notable example, crucial for antioxidant defenses in aerobic organisms.
Milk peptides are another class, derived from milk proteins either through enzymatic breakdown by digestive enzymes or by proteinases from lactobacilli during milk fermentation. Peptones, which are peptides obtained from the proteolytic digestion of animal milk or meat, are used in laboratories as growth nutrients for bacteria and fungi.
Additionally, peptide fragments are typically the result of enzymatic degradation in controlled laboratory settings, but they can also occur naturally due to environmental degradation. These classifications highlight the diverse origins and functions of peptides in biological systems.
LifeLinkResearch.com uses cookies to enhance your experience. By continuing to use our website, you consent to our use of cookies in accordance with our Cookie Policy.
You confirm having read our waiver and disclaimer of liability. By using this site, you agree to all terms herein.
Products on this site are intended only for research and development; they are not for human consumption and that I am a qualified individual 21 years and older. Statements and products on this site have not been evaluated by the U.S. Food and Drug Administration. Your continued use of this site implies understanding and acceptance of these conditions.
ACCEPT